上海金畔生物科技有限公司代理AAT Bioquest荧光染料全线产品,欢迎访问AAT Bioquest荧光染料官网了解更多信息。
Portelite 快速荧光法内毒素检测试剂盒
| 货号 | 60009 | 存储条件 | Multiple |
| 规格 | 500 Tests | 价格 | 17628 |
| Ex (nm) | Em (nm) | ||
| 分子量 | 溶剂 | ||
| 产品详细介绍 | |||
简要概述
产品基本信息
货号:60009
产品名称:Portelite 快速荧光法内毒素检测试剂盒
规格:500 Tests
储存条件:-15℃避光防潮
保质期:24个月
试剂盒组分
组分A:Endotoxin Green(1瓶)
组分B:不含内毒素溶液(1瓶,20ml)
组分C:Li变形细胞(1瓶)
组分D:大肠杆菌内毒素标准品(10 EU)
组分E:DMSO(1瓶,100ul)
适用仪器
| CytoCite 荧光计 | |
| 激发: | 480nm |
| 发射: | 510-580nm |
| 器材: | 0.2 mL薄壁PCR管 |
产品介绍
脂多糖(LPS),也称为内毒素,是革兰氏阴性细菌外膜的主要成分。 LPS是脊椎动物先天免疫系统的有效刺激剂,可引起发烧,败血性休克并最终导致死亡。 LPS还被认为是检测细菌病原体入侵的生物标记,在极端情况下还负责炎症反应和内毒素休克的发展。检测生物材料(例如蛋白质,肽或抗体样品)中的LPS是生物制造和加工中的关键任务。 Portelite 快速荧光内毒素检测试剂盒使用Endotoxin Green (一种敏感的荧光底物)。Endotoxin Green可以在存在内毒素和血红细胞裂解液(LAL)(一种来自crab蟹的血细胞的提取物)的情况下进行水解,产生强烈的绿色荧光。内毒素活性与Endotoxin Green 水解产生的荧光强度成正比。该试剂盒针对Cytocite 和Qubit 荧光计进行了优化,可以检测样品中存在的多种内毒素(从1 EU / ml到0.002EU / ml)。金畔生物是AAT Bioquest的中国代理商,为您提供最优质的Portelite 快速荧光法内毒素检测试剂盒。
图示
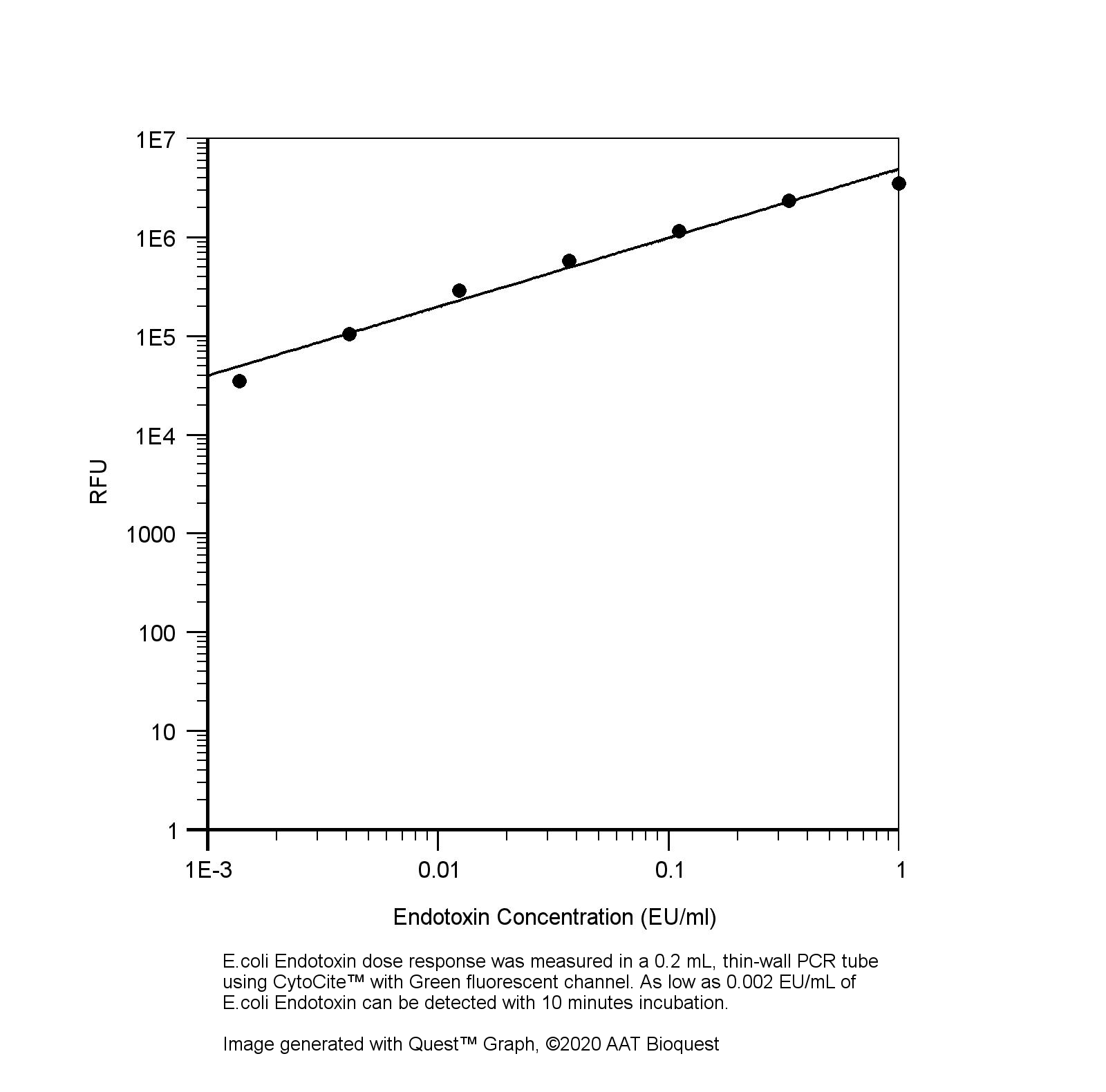 图1. Portelite 快速荧光内毒素检测试剂盒图 |
参考文献
A Validation Study of the Limulus Amebocyte Lysate Test as an End-Product Endotoxin Test for Polyvalent Horse Snake Antivenom
Authors: Sheraba, N. S., Diab, M. R., Yassin, A. S., Amin, M. A., Zedan, H. H.
Journal: PDA J Pharm Sci Technol (2019): 562-571
Utility Of An Automatic Limulus Amebocyte Lysate Kinetic Turbidimetric Test For Endotoxin Screening Of Dialysate Samples
Authors: Uchida, T., Kaku, Y., Hayasaka, H., Kofuji, M., Momose, N., Miyazawa, H., Ueda, Y., Ito, K., Ookawara, S., Morishita, Y.
Journal: Med Devices (Auckl) (2019): 429-433
COMPARISON OF QuantiFERON(R) TB GOLD TEST RESULTS BEFORE AND AFTER ENDOTOXIN CONTAMINATION
Authors: Seto, J., Suzuki, Y., Ahiko, T.
Journal: Kekkaku (2016): 49-52
Detection of Endotoxin Contamination of Graphene Based Materials Using the TNF-alpha Expression Test and Guidelines for Endotoxin-Free Graphene Oxide Production
Authors: Mukherjee, S. P., Lozano, N., Kucki, M., Del Rio-Castillo, A. E., Newman, L., Vazquez, E., Kostarelos, K., Wick, P., Fadeel, B.
Journal: PLoS One (2016): e0166816
Evaluation of a portable test system for assessing endotoxin activity in raw milk
Authors: Suzuki, Y., Suzuki, K., Shimamori, T., Tsuchiya, M., Niehaus, A., Lakritz, J.
Journal: J Vet Med Sci (2016): 49-53
Evaluation of the endotoxin binding efficiency of clay minerals using the Limulus Amebocyte lysate test: an in vitro study
Authors: Schaumberger, S., Ladinig, A., Reisinger, N., Ritzmann, M., Schatzmayr, G.
Journal: AMB Express (2014): 1
Comparison of Limulus amebocyte lysate test methods for endotoxin measurement in protein solutions
Authors: Chen, L., Mozier, N.
Journal: J Pharm Biomed Anal (2013): 180-5
Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test
Authors: Hasiwa, N., Daneshian, M., Bruegger, P., Fennrich, S., Hochadel, A., Hoffmann, S., Rivera-Mariani, F. E., Rockel, C., Schindler, S., Spreitzer, I., Stoppelkamp, S., Vysyaraju, K., Hartung, T.
Journal: ALTEX (2013): 169-208
Contamination of nanoparticles by endotoxin: evaluation of different test methods
Authors: Smulders, S., Kaiser, J. P., Zuin, S., Van L and uyt, K. L., Golanski, L., Vanoirbeek, J., Wick, P., Hoet, P. H.
Journal: Part Fibre Toxicol (2012): 41
Gel clot bacterial endotoxin test of FDG: Indian scenario
Authors: Sharma, S., Mittal, B. R., Vatsa, R., Singh, B.
Journal: Indian J Nucl Med (2011): 149-52
