Endoglycoceramidase I (EGCase I) 收藏










Download:
识别位点
- isoschizomers |
- compatible ends |
- single letter code
特性
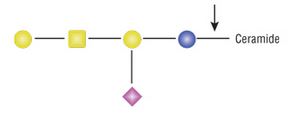
Catalyzes the hydrolysis of the β-glycosidic linkage between oligosaccharides and ceramides in
various glycosphingolipids.
Oligosaccharide and ceramide remain intact, leaving them suitable for further analysis.
This is an Enzyme for Innovation (EFI). EFI is a project initiated by NEB to provide unique enzymes to
the scientific community in the hopes of enabling the discovery of new and innovative applications.
These enzymes have interesting properties and unique specificities.
概述
Endoglycoceramidase I (EGCase I) catalyzes the hydrolysis of the β-glycosidic linkage between
oligosaccharides and ceramides in various glycosphingolipids. The enzyme leaves the oligosaccharide
and ceramide intact, keeping them suitable for further analysis. The enzyme efficiently releases
oligosaccharides from ganglio- and globo-type glycosphingolipids, certain type of cerebrosides
(glucosylcerebrosides), and exhibits low activity towards galactocerebrosides (See Reference 1,
FAQ #3). EGCase I does not hydrolyze sulfatides nor psychosine.
来源
EGCase I is isolated from a strain of E. coli, which contains the cloned EGCase I gene from Rhodococcus triatomea.
反应条件
1X EGCase I Reaction Buffer. Incubate at 37°C
随酶提供
10X EGCase I Reaction Buffer
1X EGCase I Reaction Buffer
0.1% Triton® X-100
50 mM sodium acetate
(pH 5.2 @ 25°C)
分子量
Apparent: 50 kDa
单位定义
One unit of R. triatomea EGCase I was defined as the amount of enzyme required to hydrolyze 1 μmol of
ganglioside GM1a per minute at 37°C.
Unit Assay Conditions
Two fold dilutions of EGCase I are incubated with 10 nmoles of GM1a substrate in 1X EGCase I Reaction
Buffer in a 10 µl reaction. The reaction mix is incubated for 5 minutes at 37°C. Released glycans are
labeled with 2-AB, and reaction products are analyzed by ultra-performance hydrophilic interaction liquid
chromatography with fluorescence detection.
热失活
65°C for 20 min.
参考文献
1. Albrecht, S. et al. (2016). Analytical Chemistry. 88, 4795-4802.